Harnessing CAR-T Therapy: A New Frontier in Lupus Treatment
Written on
Chapter 1: Understanding Systemic Lupus Erythematosus
For many teenagers, life revolves around simple aspirations like career choices and pets. Uresa, however, faced a challenging reality as her immune system spiraled out of control, leading to a diagnosis of systemic lupus erythematosus (SLE). This condition is characterized by the immune system erroneously attacking various organs and tissues, resulting in widespread inflammation and damage. Affected areas can include the joints, skin, kidneys, and even the brain. Due to the absence of a cure, treatment typically focuses on managing symptoms and preventing disease progression.
SLE often develops slowly, commonly diagnosed around age 31, and is seldom seen in children. However, when it manifests at a young age, as in Uresa's case, it tends to be more severe. By the age of 15, she exhibited significant symptoms, including debilitating headaches, fatigue, joint pain, and the hallmark butterfly rash.
Despite various treatments ranging from mild immunosuppressants to chemotherapy, Uresa continued to suffer from high fevers, anemia, and dangerously elevated levels of autoantibodies. As a result, she required daily plasmapheresis, a procedure to remove harmful components from her blood. Tragically, within a year, the toxic effects of the medications severely damaged her kidneys, leading her to need hemodialysis. Her SLEDAI score, which gauges disease activity, soared to an alarming 23, indicating extreme severity.
In a desperate move, Dr. Krickau, her pediatric rheumatologist, suggested exploring CAR-T cell therapy as a last resort.
Section 1.1: The Science Behind CAR-T Therapy
CAR-T therapy represents a groundbreaking approach where the body’s immune cells are harnessed to combat diseases like cancer. Our immune system relies on red and white blood cells; while red blood cells transport nutrients, white blood cells serve as defenders against various threats.
Among these, T cells and B cells are crucial for combating complex issues. B cells produce antibodies that tag harmful invaders, while T cells actively seek and destroy aberrant cells, including those infected by viruses or cancerous cells.
In the early 2000s, researchers began utilizing T cells in the treatment of specific blood cancers. The process involves extracting T cells from a patient, modifying them to recognize cancer-specific markers, amplifying their numbers, and reinfusing them. The modified T cells, now equipped with chimeric antigen receptors (CAR), function as living medications, targeting and eliminating cancer cells.
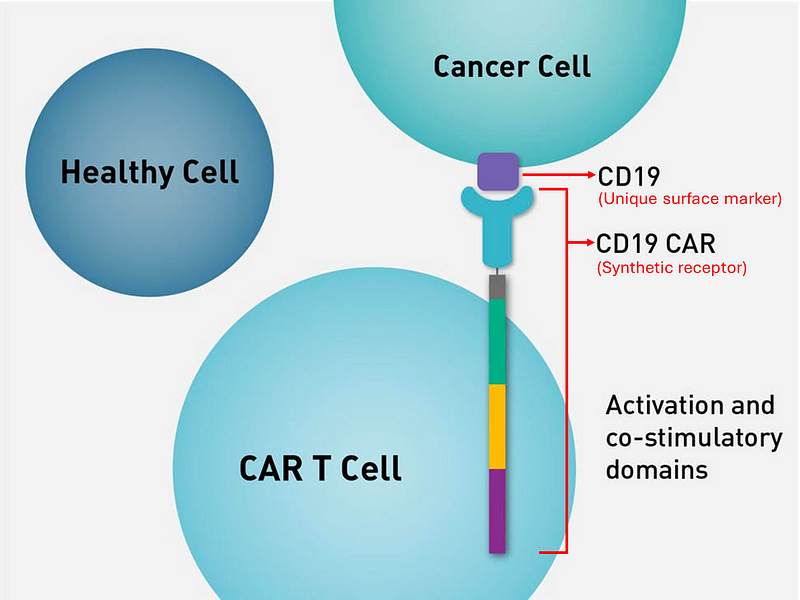
Section 1.2: CAR-T Therapy's Link to Lupus
Currently, CAR-T therapy is FDA-approved primarily for specific blood cancers due to its engineered ability to target B cell markers. However, given that abnormal B cells are a defining characteristic of SLE, CAR-T cells could potentially provide therapeutic benefits for lupus patients.
In a 2022 study, eight adults with SLE who had not responded to conventional treatments participated in a compassionate-use CAR-T program. Remarkably, after undergoing CAR-T therapy, five patients achieved a SLEDAI score of zero within three months, and their autoantibodies vanished. Their kidney functions also normalized, and some maintained remission for up to 29 months.
Nonetheless, the use of CAR-T therapy in children like Uresa raised concerns about potential side effects. Dr. Krickau expressed apprehensions regarding administering modified immune cells to a minor, as such treatments had never been attempted in children.
Chapter 2: Uresa's Journey with CAR-T Therapy
In June 2023, Uresa began her CAR-T treatment after a five-day course of mild chemotherapy designed to prepare her immune system. The results were astonishing: her B cell count plummeted to zero, her autoantibodies disappeared, and her symptoms resolved. Her kidneys, previously damaged, showed significant recovery by the third week post-treatment.
Dr. Krickau and the medical team classified Uresa’s outcome as a “dialysis-free, partial renal response,” a result they had not anticipated given her previous condition. By the end of July 2023, Uresa returned home, ready to continue her education. “I feel as good as I did before my diagnosis,” she remarked, save for a few minor illnesses.
Section 2.1: Risks and Considerations of CAR-T Therapy
While CAR-T therapy holds great promise, it is not devoid of risks. One notable side effect is cytokine release syndrome (CRS), which occurs when a large number of T cells are activated, leading to an influx of cytokines and resulting in inflammation. Reports indicate that a significant percentage of patients undergoing CAR-T therapy experience varying degrees of CRS, raising concerns for individuals with SLE who already endure chronic inflammation.
Neurotoxicity is another risk, manifesting through symptoms such as confusion and difficulty with attention. Data shows that a considerable number of patients treated for blood cancers reported these adverse effects.
Conclusion: The Path Forward for CAR-T Therapy in Lupus
In conclusion, CAR-T therapy represents a significant advancement in the treatment of severe SLE, a condition that has long lacked effective solutions. While early results from adult studies and Uresa’s case bring hope, it remains crucial to further refine the treatment to mitigate risks. Ongoing research into CAR-T therapy for lupus is promising, with numerous clinical trials currently being conducted worldwide.
As of September 9, 2024, 43 studies are registered, with 30 actively recruiting participants globally, including in China, the U.S., and Europe. With continued advancements, CAR-T therapy may soon become a viable treatment option for lupus.
Author’s Note: A heartfelt thank you to the reader who shared Uresa’s inspiring story with me, motivating this article. For more insights on lupus, feel free to explore additional posts. If you appreciate my work, consider subscribing to my Medium email list or offering financial support. Your contribution is greatly valued.