Nature's Astonishing Discovery: The Oklo Natural Nuclear Reactor
Written on
Chapter 1: A Geological Marvel
Deep within Africa lies a fascinating nuclear mystery. Picture the year 1956: the Cold War is quietly intensifying, and Elvis Presley's "Blue Suede Shoes" fills the airwaves. Nations around the globe are vying for access to Uranium 235, a key element in nuclear armament. France, controlling the region of Gabon, stumbles upon the Oklo area, abundant in Uranium ore. However, the extracted Uranium seemed unusual, as if it had already been subjected to reactor conditions. Had someone preempted them? As it turns out, they uncovered one of the most extraordinary geological formations: a two-billion-year-old natural nuclear reactor.
Uranium exists in various isotopes, but Uranium 235 (²³?U) is the one sought after for reactors and atomic bombs. With a half-life of 700 million years, this isotope undergoes radioactive decay into Thorium 231 over vast time periods. Yet, when bombarded with slow-moving neutrons, ²³?U can undergo rapid decay, initiating a chain reaction known as fission. This unique property makes ²³?U invaluable.
Fortunately, there is a natural source of neutron radiation, but these fast-moving particles need to be moderated to effectively interact with ²³?U. By immersing the Uranium in a control medium like water, neutrons are slowed enough to be absorbed by ²³?U. This absorption transforms it into a new isotope, which is highly unstable and quickly decays into Barium 141 (¹?¹B), Krypton 92 (?²Kr), and three energetic neutrons. If these neutrons are subsequently slowed down, they can trigger further decays in additional ²³?U atoms, leading to a runaway fission chain reaction.
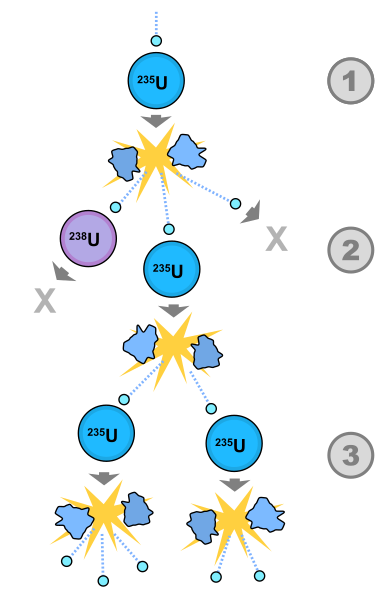
This fission chain reaction is the principle behind nuclear power plants and bombs—essentially a domino effect of radioactivity. Much like carbon dating, scientists can estimate the expected ratio of ²³?U in Uranium ore based on its age. For instance, if the ore is 700 million years old, it should contain half the original ²³?U amount.
All Uranium on Earth originated at the same time—about six billion years ago when a precursor star to our Sun went supernova. This means that Earth's Uranium should currently have a concentration of 0.72% ²³?U. Surprisingly, French scientists discovered a concentration of just 0.717%.
Although this discrepancy seems minor, Oklo contained a vast quantity of Uranium—equivalent to 200 kg of ²³?U, enough to construct six atomic bombs! Naturally, this raised concerns amidst the political climate of the time, where nations were heavily investing in nuclear arsenals. French officials feared that their ²³?U source had been compromised, which could lead to significant international tensions.
Upon closer examination, scientists found substantial decay byproducts of ²³?U, like ?²Kr and ¹?¹B, indicating that the Uranium had already undergone fission in a reactor before being buried again. This revelation was perplexing.
Years prior to mining, scientists hypothesized the existence of a geological structure capable of functioning as a naturally regulated nuclear reactor. Further investigations confirmed that Oklo met all necessary geological criteria, marking it as the first known natural nuclear reactor.
Section 1.1: How Natural Reactors Operate
But how does such a naturally occurring reactor function? And why didn't it go supercritical and explode? This reactor was active approximately two billion years ago during the Paleoproterozoic era, a time when multicellular life was just emerging. Back then, the concentration of ²³?U was about 3%, similar to that found in modern reactors.
One challenge remains: without a means to slow down the neutrons, criticality cannot be achieved. Neutrons from natural ²³?U decay tend to escape without interacting with other ²³?U atoms. Thus, a moderator is essential to enable the neutrons to attach to ²³?U and initiate a chain reaction.
Fortunately, the Oklo reactor was blessed with a natural moderator in the form of a water table. The Uranium ore was submerged in a continuously flowing subterranean waterway, which slowed down neutrons, enabling the ore to reach criticality and sustain a chain reaction.
Now, if the water were to remain static, the reaction could escalate and become supercritical—a phenomenon where fission occurs exponentially, akin to a nuclear bomb. However, Oklo was uniquely structured to prevent such a runaway reaction; the water not only initiated the chain reaction but also regulated it effectively.
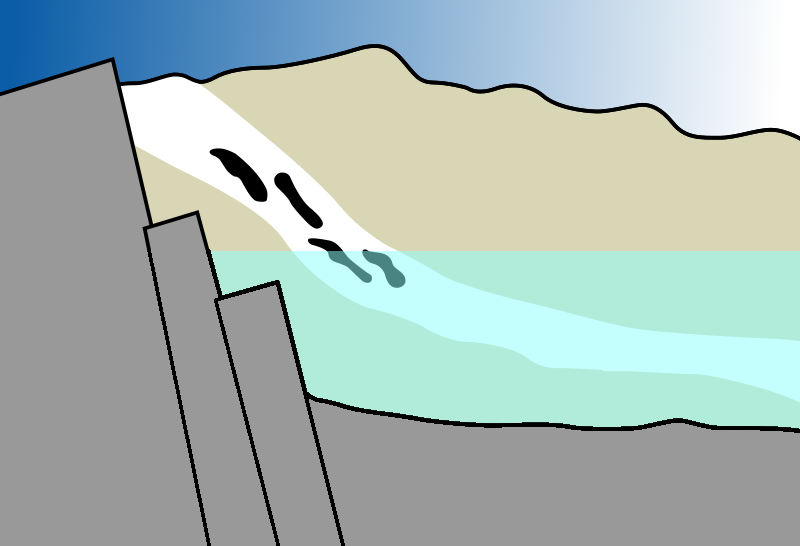
In the accompanying diagram, you can see a cross-section of the Oklo reactor. As water flows through, it slows down the neutrons, causing the ore to heat up. However, after about thirty minutes, the water becomes so hot that it vaporizes, ceasing to moderate the neutrons and halting the reaction. Once the reactor cools and water returns, the chain reaction resumes. This cycle persisted for hundreds of thousands of years until the ²³?U concentration diminished too much to sustain criticality.
Thanks to this unique water control, Oklo was never in danger of a nuclear meltdown. Nonetheless, it managed to produce a significant amount of energy—averaging around 100 kW of heat energy, comparable to that generated by 1,000 incandescent light bulbs or slightly more than a Delorean at full speed. Remarkably, this continued for hundreds of thousands of years!

This intriguing phenomenon highlights just how astonishingly complex nature can be. Now that we understand the existence of such reactors, it opens up the possibility that other regions of the universe may host similar geological wonders. It’s not difficult to envision distant planets with their own natural reactors powering hydrothermal vent-like structures. Such environments could potentially support primitive life forms capable of thriving in radiation-rich, heated waters. Imagine that—a world where bacteria thrive on nuclear energy!
Chapter 2: Understanding the Oklo Reactor
The first video titled "How Mother Nature Built a Nuclear Reactor Called Oklo" explores the incredible natural reactor and its implications for science and geology.
The second video titled "Natural Nuclear Reactor" delves into the science behind natural reactors and their significance in our understanding of nuclear energy.