Exciting Breakthrough: Universal T-cell Receptor for Cancer Treatment
Written on
Chapter 1: Introduction to Immunotherapy
Recent advancements have led to significant breakthroughs in cancer treatment, particularly through immunotherapy. This method aims to harness the body’s immune system to combat the disease. A notable study published last year explored a technique where T-cells are extracted from a patient, reprogrammed to target specific cancer cells, and then reintroduced into the body. This innovative approach was so impactful that it garnered the 2018 Nobel Prize in Medicine.
Section 1.1: The Limitations of CAR-T Therapy
Currently, the most prevalent FDA-approved treatment, CAR-T immunotherapy, is a highly personalized strategy that focuses on specific proteins present on cancer cells. However, it is hindered by several drawbacks, including high costs, lengthy preparation times, and potential side effects. A significant challenge with CAR-T therapy has been the lack of a universal T-cell receptor (TCR) that could effectively target various cancers in all patients.
Subsection 1.1.1: A Groundbreaking Discovery
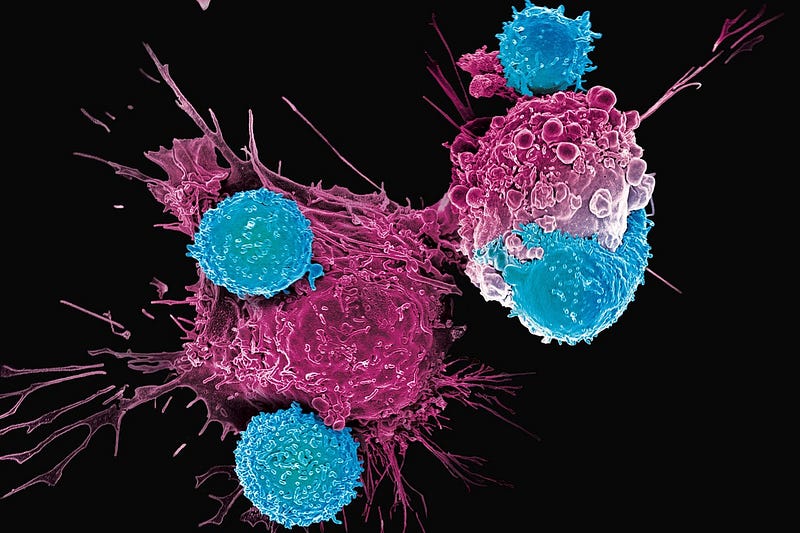
Researchers from Cardiff University have challenged this belief with the identification of a revolutionary universal killer T-cell. This newly uncovered T-cell receptor (TCR) has the capability to act as a universal agent against a wide array of human cancer types while sparing healthy cells.
“Targeting cancer through MR1-restricted T-cells opens up exciting possibilities for a ‘one-size-fits-all’ treatment approach; a single type of T-cell could potentially eliminate various cancer types across different individuals. This was previously thought impossible.” ~ Andrew Sewell, Lead Author
Section 1.2: Mechanism of Action
The mechanism involves the TCR binding to a surface molecule known as MR1, which is found on the exterior of cancer cells. While this molecule is present in nearly all human cells, its unique configuration on cancer cells allows the TCR to differentiate between malignant and healthy cells effectively. Laboratory tests have confirmed the successful targeting of several cancer types, including lung, skin, blood, colon, breast, bone, prostate, ovarian, kidney, and cervical cancers.
Chapter 2: Promising Early Trials
Initial trials conducted on mice have yielded encouraging results. Researchers injected T-cells that recognized MR1 into mice with human cancers and functioning human immune systems. The outcomes of these trials showed cancer-clearing effects comparable to those of the established CAR-T immunotherapy.
The implications of this study could be transformative for cancer treatment. Ongoing research aims to clarify how the TCR identifies healthy cells versus cancerous ones. The research team anticipates commencing human trials by the end of this year to validate their findings and ensure safety.
The full research details were published in the journal Nature Immunotherapy. Stay updated with essential information—subscribe to my mailing list.