Unlocking Mars: Comet-Inspired Innovations for Terraforming
Written on
Chapter 1: The Challenge of Mars' Atmosphere
Mars presents a formidable obstacle for colonization due to its thin atmosphere, which is primarily composed of carbon dioxide. This lack of breathable oxygen means that any potential settlers would remain confined indoors or reliant on spacesuits. However, groundbreaking research from Caltech, inspired by the behavior of comets, suggests a promising method for converting the carbon dioxide in Mars' atmosphere into breathable oxygen.
Breathable oxygen is critical for human space exploration. The oxygen we inhale is made up of pairs of oxygen atoms (O2), whereas carbon dioxide (CO2) consists of two oxygen atoms flanking a single carbon atom.

As humanity begins to establish a presence on Mars, the necessity of either transporting air or being confined to habitats becomes clear. Image credit: NASA
Konstantinos Giapis, a chemical engineering professor at Caltech, noted, “We initially believed it was impossible to bond the two oxygen atoms within a CO2 molecule since CO2 is a linear structure, which would require significant deformation to achieve this.”
Astronomers have long suspected that molecular oxygen is present in comet tails, though the exact process that produces this gas has remained unclear. Giapis and his team sought to explore this mystery, proposing that an unknown chemical reaction could be responsible.
Section 1.1: The Role of Kinetic Energy
Most chemical reactions typically require heat to initiate. Giapis demonstrated that certain reactions could harness the kinetic energy of moving molecules to trigger reactions. When water molecules collide with materials containing oxygen, such as sand or rust, they can disassociate, resulting in the formation of molecular oxygen and hydrogen. This phenomenon occurs near comets, where water vapor released as a comet nears the Sun gets propelled backward by solar winds, eventually colliding with the comet’s frozen surface.
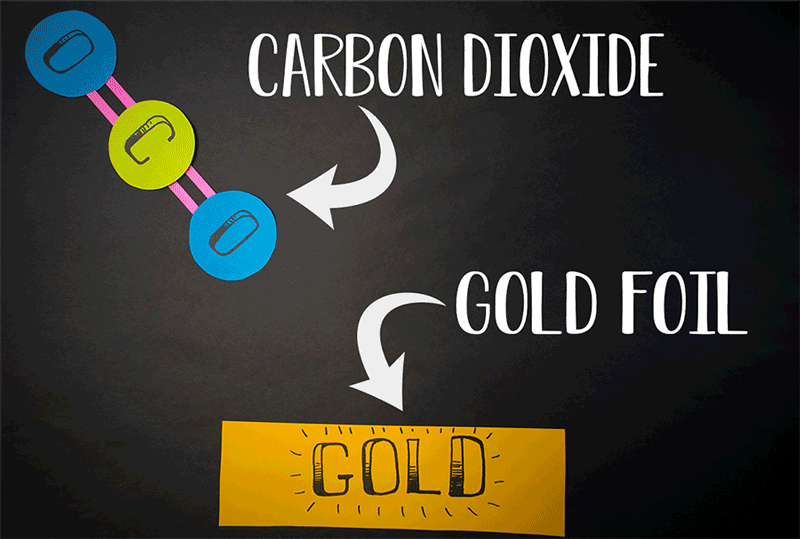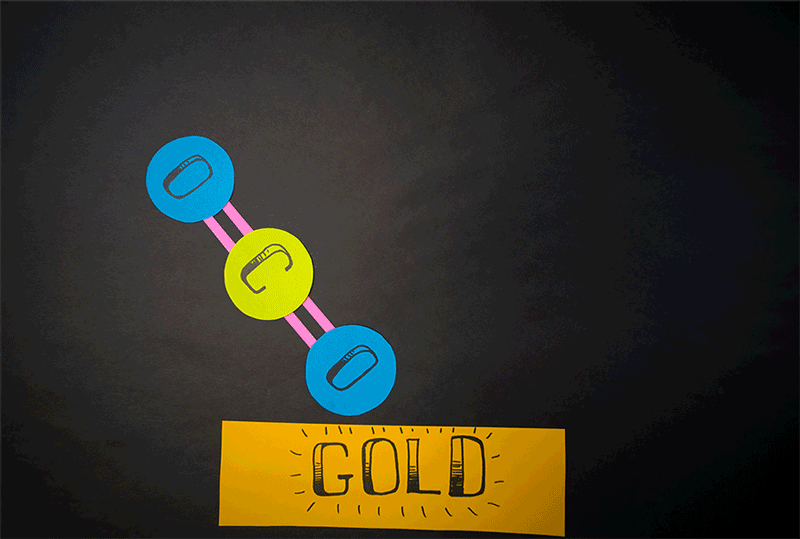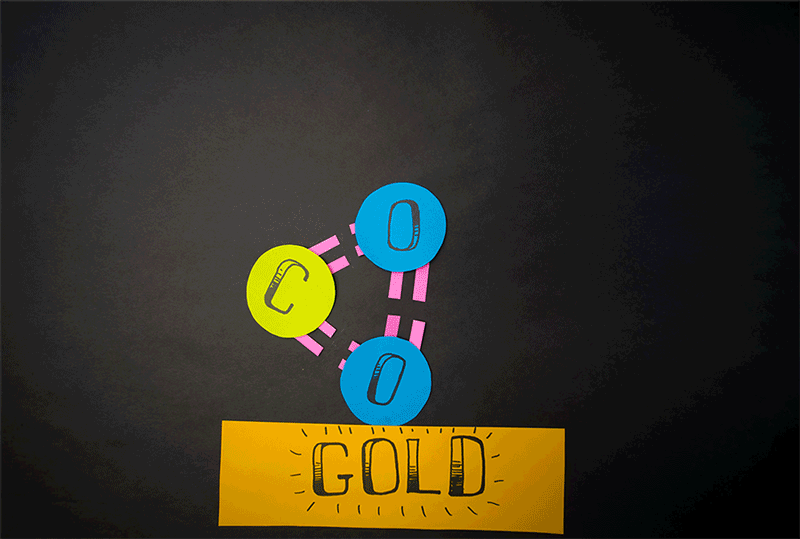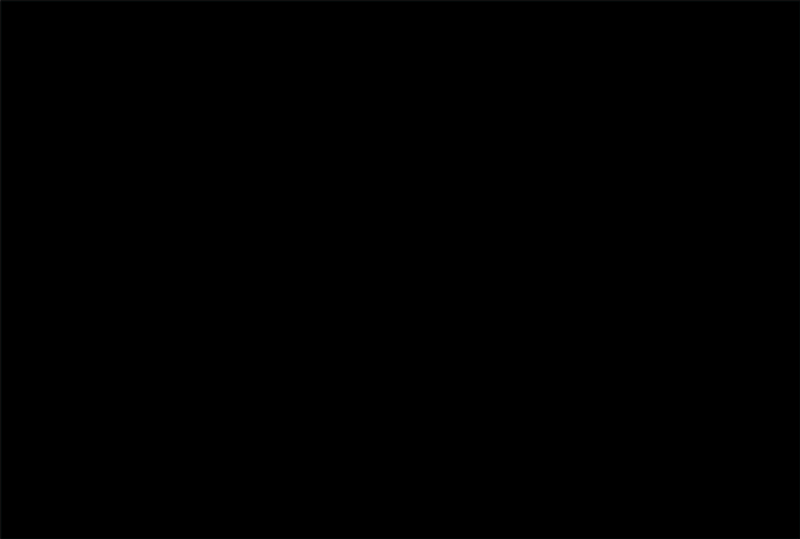
Giapis and his team accelerated CO2 molecules onto a gold plate and observed that a small percentage of the carbon dioxide was transformed into breathable oxygen.
They created a device that energizes the gas, ionizing the molecules, and then propelling them through an electric field towards a target for collision. This innovative approach, which could signify a new chemistry branch, doesn't necessitate extreme speeds for molecular conversion, as their findings indicate.
“You could launch a stone at sufficient velocity at CO2 to achieve a similar effect. It would need to travel as fast as a comet or asteroid in space,” Giapis explained.
Computer simulations were constructed to replicate the experiment; however, the chaotic nature of molecular movements presented significant challenges.
Researchers believe a similar process might already be occurring in Mars' upper atmosphere, where high-speed dust particles collide with carbon dioxide molecules, potentially explaining the presence of oxygen in that region.
"Curiosity and exploration are fundamental to human nature. When we cease these pursuits, we cease to be human. I've experienced more in my lifetime than I ever anticipated. Many of Earth's challenges can only be addressed through space technology, and our next steps lie in the cosmos. It's unavoidable." — Arthur C. Clarke
Chapter 2: Preparing for Mars Missions
As we contemplate human missions to Mars, the logistics of transporting oxygen become a significant concern, as it considerably increases the mission's weight and cost. The new Caltech device produces only one to two percent of breathable oxygen from the CO2 it interacts with, limiting its immediate utility for space missions. Nevertheless, the technology unveiled here could eventually lead to the development of systems capable of generating substantial oxygen quantities from carbon dioxide.
“Establishing sustainable human life on another planet will demand vast resources, which we cannot transport entirely. We must innovate. If we can convert abundant resources like carbon dioxide into various useful products, the possibilities for both space and Earth applications are endless,” stated Monsi Roman, program manager for NASA’s Centennial Challenges.
Beyond providing air for astronauts and potential space settlers, understanding this conversion process could yield direct benefits for Earth, especially in developing technologies to remove carbon dioxide from the atmosphere.
With implications for addressing global climate change, aiding human exploration of space, and possibly terraforming Mars, this new discovery holds immense promise for the future of humanity—on Earth and beyond.