When Dead Scientists Reflect: Regrets Across Generations
Written on
The Historical Echoes of Regret
"Those who do not learn from history are doomed to repeat it." — Winston Churchill
In the realm of business, the pursuit of profit is a universally recognized principle. To fuel growth, companies constantly seek ways to enhance efficiency and, in turn, increase their earnings. The logic is simple: by reducing operational costs while maintaining prices, more revenue can flow into the business. During the early 1800s, entrepreneurs began to view the emerging field of chemistry as a promising avenue for improving efficiency and profitability. The success of these scientist-entrepreneurs ultimately drew the attention of governments, leading to increased investments in chemistry. This is precisely how Germany emerged as a leading industrial and academic force in the 19th century—a narrative worth exploring in its own right.
One sector that greatly benefited from advancements in chemistry was mining. From the salt mines of Carthage to the coal mines of West Virginia, mining is an arduous and labor-intensive endeavor. Consequently, the industry was heavily focused on minimizing factors that hinder efficiency. Accessible ore veins were essential; even a lucrative vein would be abandoned if it required excessive effort to reach. Innovations that could reduce the substantial labor involved while improving access to ore would have a transformative impact on both the industry and society at large.
This context presented a ripe opportunity for enterprising chemists. One notable figure, who came from humble beginnings and had experienced several business failures before achieving success, recognized this potential. Through his newfound wealth, he dedicated himself to studying chemistry, mastering an impressive array of languages, and exploring various industries to determine how chemistry could facilitate business growth.
His focus eventually led him to the mining sector and the chemistry of explosives. Until that time, black powder was the standard explosive used in mining, but it proved inadequate. To understand why, we must delve into the chemistry and physics of explosives.
The speed of an explosive reaction is paramount—not in terms of how fast the reaction occurs but rather the speed of the flame produced by it. This may sound abstract, but a rapidly moving flame generates a pressure wave, which is the primary cause of damage in an explosion and a key focus for researchers developing new explosives. The distinction between deflagration and detonation hinges on the flame front's velocity: deflagrations are slow, while detonations are fast. The term "high explosives" refers to materials that cause detonations, while "low explosives" denote deflagrations.
Both types of explosives serve important purposes, and just because a reaction results in a deflagration doesn't mean it lacks utility or safety.
Turning back to our narrative, the mining industry was largely reliant on black powder, a slow explosive that did little to move vast amounts of rock effectively. While black powder was suitable for propelling bullets, its weaker pressure waves often failed to break rock effectively. An alternative, nitroglycerin, was known for its extreme sensitivity and danger, making it nearly unmanageable despite its extraordinary power for displacing large volumes of rock. Mining companies tolerated the risks associated with nitroglycerin, as other industries found it far too perilous.
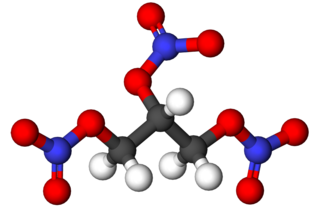
"Ball and stick" model of nitroglycerin — colored spheres represent atoms (white = hydrogen, gray = carbon, red = oxygen, blue = nitrogen), with connections signifying chemical bonds.
Recognizing the opportunity, our chemist was determined to create a safer version of nitroglycerin without compromising its effectiveness. His breakthrough came when he discovered that mixing nitroglycerin with diatomaceous earth significantly decreased its sensitivity to shock. For those unfamiliar, diatomaceous earth is composed of the crushed remains of diatoms (a type of plankton). It appears as a powder, but its porous structure plays a crucial role in stabilizing nitroglycerin by preventing molecules from colliding with one another during movement.
Imagine a scenario where a group of intoxicated individuals begins to vomit: if one person throws up in a shared space, it might trigger a chain reaction. However, if that person is isolated in a bathroom, the others remain unaffected.
The chemist behind this innovation was Alfred Nobel, the same Nobel known for establishing the Nobel Prize. His creation of a stabilized form of nitroglycerin, which he called dynamite, along with a safe detonation method, revolutionized the mining industry by allowing for safer access to deeper ore veins. However, the repercussions of his inventions would haunt him throughout his life.
The military quickly recognized the potential of Nobel's dynamite for causing destruction with reduced risk to their own forces. Consequently, European militaries became significant clients of his explosive. Witnessing the devastation wrought by his invention filled Nobel with guilt, prompting him to use the wealth he earned to establish the Nobel Prize as a means of seeking redemption.
Meanwhile, nearly a century later, another scientist would find himself in a similar predicament. As the war in Europe began to wind down, the conflict in the Pacific persisted. The Allies sought a way to end hostilities without launching a full-scale invasion of Japan. One solution emerged in the form of a weapon exponentially more potent than dynamite: the atomic bomb. Following the successful detonation of a test bomb on July 16, 1945, the world entered the nuclear age. It’s easy to imagine Nobel lamenting the path humanity had taken.
The lead scientist of the Manhattan Project, Robert Oppenheimer, would later advocate against the weapon he had helped create, feeling an immense sense of guilt over its destructive potential. His regret was so profound that it strained his relationship with President Truman, who famously dismissed Oppenheimer's concerns.
The parallels between Nobel and Oppenheimer are striking. Both scientists aspired to use their work for the betterment of humanity, yet both inadvertently contributed to the creation of tools that inflicted harm. Their legacies are marked by profound regret for the unintended consequences of their inventions. If they could converse, what wisdom might they share, perhaps echoing Churchill's warning about the lessons of history?